Hydrogen combustion for decarbonization of (high-temperature) process heat
Abstract
Climate change and decarbonisation are a significant challenge for energy-intensive industries. In many industries, energy is needed primarily as process heat at very different temperature levels for a multitude of highly specialised manufacturing processes, with high requirements for product quality, economic efficiency and environmental compatibility. Hydrogen from renewable sources presents a promising option for decarbonising these processes, but, due to its combustion properties, comes with some special aspects, which must be taken into consideration in these complex applications.
Introduction
Climate change and the resulting necessity to minimise anthropogenic greenhouse gas emissions (GHG) presents a global challenge in this century. The focus here are especially emissions associated with the supply of energy, from electricity generation in power plants through propulsion power for vehicles to heat in the residential sector, but also in many primary industries. Process heat is responsible for around 2/3 of the industrial energy consumption in Germany [1] or for around 18 % of the German greenhouse gas emissions [2].
Easily the largest part of the process heat requirement in Germany is covered today by fossil fuels, primarily natural gas (approx. 45 %) while electricity or renewable fuels play only a minor role (together around 13 %) [2]. This distribution is likely to be similar in most industrialised nations.
Process heat requirements are very heterogeneous: the necessary temperature levels range from low-temperature applications like, for instance, steam generation, to process temperatures of 1,500 °C and more, for instance in the glass industry. Moreover, in many industrial production processes, it is not only about heating the materials, at the same time other physical and chemical processes play an important part, e.g. in hardening metals, melting materials or firing ceramics. The products range from foodstuffs to everyday materials like steel, aluminium, glass, cement or ceramics.
In principle, various options are available for decarbonising process heat, for instance the removal of CO2 from the flue gas and subsequent storage or utilisation (CCUS: Carbon Capture, Utilisation and Storage), or the use of biogenic fuels like biomass or biogas. Electrification (with green electricity, see e. g. [3]) and the use of hydrogen (H2) are currently regarded as the most promising options. While, up until a few years ago, the focus was on the extensive electrification of manufacturing processes [3], [4], interest in hydrogen as a carbon-free fuel for process heat has increased considerably in recent years [5].
There are several reasons for this: first, it is often easier to switch a complex natural-gas-heated production process to a different fuel like hydrogen than to a completely new heating system like resistive electric heating [6], [7]. Moreover, electric kilns and furnaces are often limited in the quantity of transferable heat, so that they are often larger than comparable gas-heated installations [6], [8], [9], and there are processes that are either difficult to electrify or cannot be electrified at all, for instance in the glass or aluminium industries [9], [10].
Hydrogen is interesting for another reason. Today, Europe imports just under 60 % of the energy it needs in the form of coal, natural gas and oil, and it is foreseeable that in the future, too, demand will not be met solely by renewable energies in Germany and Europe [11], [12]. Global energy trading or long-term storage of large quantities of energy will also be necessary in future. It cannot, however, be realised on the required scale with electricity due to transmission losses and the low energy density of batteries. Here, hydrogen or hydrogen derivatives like ammonia (NH3) are one of the most promising options for transporting large quantities of decarbonised energy intercontinentally from producers (especially in the global south) to the big consumers in Europe and Asia [12], [13] and for compensating for seasonal variations with storage. Studies have shown that a large part of the existing, highly developed natural gas infrastructure can be converted for hydrogen at reasonable cost and effort [14], [15], which makes hydrogen interesting especially for energy-intensive high-temperature processes in industry that are particularly dependent on a reliable supply on account of their usually continuous operation.
Hydrogen vs. natural gas: a comparison of the combustion properties
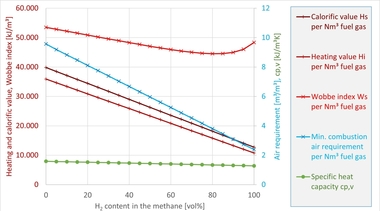
As fuel for high-temperature process heat, hydrogen offers a number of advantages. It must, however, be taken into account, that chemically it is a completely different fuel compared with natural gas, with very different properties. » Table 1 shows this based on several combustion-related characteristics for methane (CH4, representing natural gas), a mix of 80 vol% CH4 and 20 vol% H2 as well as pure hydrogen. The CH4/H2 blend has been considered here as, besides the set-up of separate H2 infrastructures like, for example, the European Hydrogen Backbone [16], the direct feed of hydrogen into existing natural gas networks has also been the subject of discussion. Various studies have shown that especially gas appliances from the residential and commercial sector can be safely operated with H2 concentrations of up to 20 vol% [17], [18]. Given the large number of installed appliances (more than 200 million in the EU [19]), the residential and commercial sector is considered especially worthy of protection. However, injecting in H2 into natural gas is not completely uncontroversial [20].
With reference to the table, some key differences between the fuels become clear. For instance, the volumetric calorific value Hi,vol of hydrogen is around a third of that of natural gas or methane, at the same time the minimum air requirement Airmin of H2 is also much lower. As a result, when the fuel is switched, the volume flows of fuel and oxidiser, with which the combustion process must be supplied, change (given the firing rate and air excess ratio are the same), as a result of which the flow in the combustion chamber and, in some cases, the mixing of fuel and oxidiser change. This can have effects, for example, on the shape of the flame, heat release or pollutant emissions, for instance with regard to nitrogen oxide emissions (NOX). The (superior) Wobbe index WS also falls with higher H2 content, although much more moderately than the calorific value or air requirement. In many gas engineering sectors, the Wobbe index is regarded as the central interchangeability criterion for gaseous fuels [21], however, it reaches its limits particularly in context of industrial combustion and in the comparison of natural gas and hydrogen.
Of special importance in the context of the combustion of H2 are the adiabatic combustion temperature Tad and the laminar combustion velocity sL. Considering Tad, it becomes clear that with the combustion of H2, much higher local temperatures must be expected in the combustion chamber than with the combustion of CH4. One potential consequence of this are therefore higher NOX emissions, as in the combustion of gaseous fuels, NOX formation is largely temperature-dependent. The formation mechanism is, however, the same in the combustion of natural gas as in H2 combustion so that many proven primary measures for NOX reduction can be modified for hydrogen combustion.
One of the clearest differences between natural gas and H2 is in the laminar combustion velocity sL, which has a significant influence on the stabilisation of flames. For hydrogen, in stoichiometric conditions, sL is a factor of 5 higher than the combustion velocity of CH4. The effects on the combustion process are, however, largely dependent on technology and relevant especially for premixed burners as there is a danger of flashback. Such burners can be found, for example, in gas heating in households or in gas heating appliances in the power plant sector. In most industrial firing systems, on the other hand, the combustion processes are mostly non-premixed combustion processes, in which, because of the operating principle, flashbacks cannot occur.
In the context of decarbonisation, the CO2 emissions produced during combustion play a central part. When burning CH4 to release 1 MJ of heat, 55 g of CO2 are formed. With the use of a CH4/H2 blend with 20 vol% H2, the CO2 emissions per MJ are reduced by around 7 %. This shows that a partial substitution of natural gas with H2 can only be an interim solution, for maximised decarbonisation, the use of approximately pure H2 is necessary. Here, the origin of the hydrogen is crucial as with the use of H2 produced in the conventional way, i.e. by means of steam reforming of natural gas, the emissions are only shifted from the firing process to the hydrogen production, and ultimately about 60 % more greenhouse gas emissions are produced than if the natural gas were burned directly. Only the use of H2 produced by means of low-greenhouse-gas production routes like steam reforming + carbon capture and storage (“blue” hydrogen) or water electrolysis with electricity from renewable sources (“green” hydrogen) presents a contribution to decarbonisation. The specific emissions given in the table refer, however, exclusively to CO2 emissions caused by the actual combustion process itself.
What effects a fuel change has is not only dependent on the fuels themselves, but also on the specific application and the combustion technology used. For instance, a residential gas heating appliance setting will react differently than an industrial high-temperature thermal processing plant like a glass melting furnace, a tunnel kiln in the clay brick and tile industry or a gas turbine in a power plant. The requirements, too, differ depending on the specific application: while for residential gas heating appliances, the focus tends to be on carbon monoxide emissions (CO) and flame stability, for industrial-scale firing processes, aspects such as product quality, energy efficiency and nitrogen oxides emissions (NOX) are generally the priority.
Hydrogen for the supply of (high-temperature) process heat
Besides applications in the chemical industry and the mobility sector, hydrogen is regarded as an interesting decarbonisation option particularly for high temperature process heating. Main features of these processes are considerable complexity and heterogeneity, with high requirements for product quality, efficiency and harmful emissions.
At present, numerous research projects are underway nationally and internationally, in which the applicability of hydrogen for process heating in energy-intensive industries is being tested. One example is the “HyGlass” project supported by the state of North Rhine Westphalia [22], [23], which investigated these aspects in the context of the glass industry. The focus was, on the one hand, on the switch from natural gas to natural gas-hydrogen blends or pure hydrogen for typical firing processes in the glass industry and, on the other hand, on aspects concerning product quality and supply with hydrogen.
The glass industry is one of the energy-intensive basic materials industries, the largest part of the energy consumption (and therefore the greenhouse gas emissions) being caused by the melting process. Natural gas is easily the most important fuel for this industry. This is one of the reasons making hydrogen interesting for the glass industry as it is often easier to switch from one gaseous fuel to another than to a completely different technology like, for example, electric heating. Electrically heated melting furnaces exist. They are, however, limited with regard to their production rates. Moreover, limitations exist for certain glass products and grades [9].
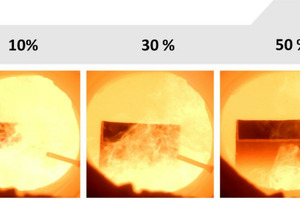
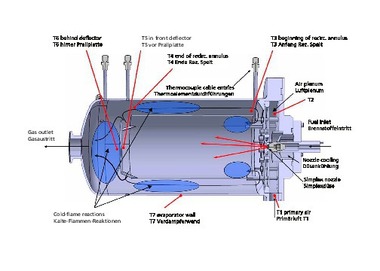
In the scope of the project, the effects of the fuel change on an underport firing, a burner configuration typical for regenerative glass melting furnaces, were investigated at one of the high-temperature burner test rigs at the Gas- und Wärme-Institut in Essen (GWI) (cf. » Fig. 1).
Here, in the test rig, boundary conditions typical for a regenerative glass melting furnace were reproduced: for instance air excess ratio (λ = 1.1), air preheating temperature (Tair = 1,150 °C) and furnace temperature (approx. 1,600 °C), at 500 kW firing rate. Real glass melting furnaces can have firing rates up to 100 MW. In the scope of the experiments, the combustion process was first set to natural gas as a reference, then the hydrogen content in the fuel gas was steadily increased. Firing rate, air excess ratio and air preheating temperature were kept constant by means of a dedicated control system. » Fig. 2 shows a view into the interior of the test rig for the different fuel gas blends.
One interesting effect of the H2 addition is that the flame becomes increasingly transparent with higher H2 content, which is associated with the change in radiation properties. With ideal lighting, an H2-flame glows pale reddish, but in normal conditions, a H2 flame is almost invisible to the naked eye, the radiation can be found essentially in the infrared and ultraviolet spectra.
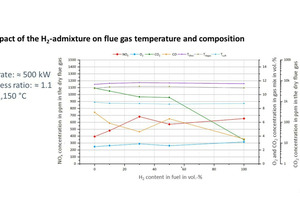
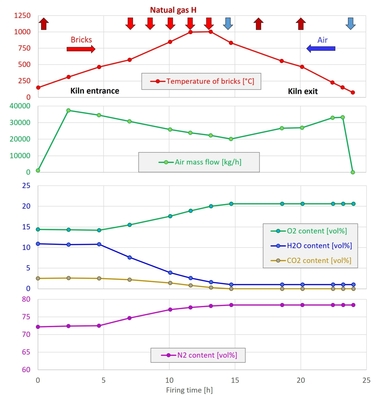
In the scope of the tests, the flue gas concentrations were recorded as shown in » Fig. 3.
In the tests, the process was controlled in such a way that despite higher H2 concentrations, the air excess ratio and the furnace chamber temperature remained constant. The diagram shows, as expected, a reduction in the CO2 concentrations with higher H2 content in the fuel, but also an increase in the NOX concentrations in the flue gas. This can be attributed to the higher local maximum temperatures in the reaction front of the flame when hydrogen is blended into the natural gas. As the formation of nitrogen oxides during the combustion of gases is generally dominated by the thermal formation path, higher temperatures in the flame lead to increased NOX formation, provided sufficient oxygen is available.
However, in the context of hydrogen combustion, “dry”-measured NOX concentrations are not the best metric for the evaluation of harmful emissions as these concentrations are determined for the dried flue gas. With H2 combustion, however, much higher concentrations of water vapour are produced than with natural gas combustion, so the measured NOx concentrations are no longer comparable [24], [25]. For instance, the measured NOX concentrations for combustion of pure hydrogen with the same boundary conditions are more than 60 % higher than for the combustion of natural gas. If, however, the emissions are referenced to the energy input, that is, for example, in the unit [mg/MJ], then only a 20-% increase in the emissions results. While a certain increase in the NOX emissions is physically reasonable on a fuel switch from natural gas to hydrogen in combustion with (preheated) air, analysis of the “HyGlass” measurements shows how the comparability of the emissions based on the use of “dry” concentrations is distorted. For the oxy-fuel combustion widely found in many high-temperature applications [26] the switch to hydrogen can even lead to much reduced NOX emissions, as the natural gas is not present as a nitrogen source, as can be proven based on CFD simulations [27] and measurements.
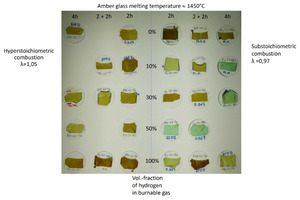
For every industrial production process, product quality is one of the most important evaluation criteria. Therefore, the impact of a switch from natural gas to natural gas/hydrogen blends and pure hydrogen on the glass quality was investigated in cooperation with the Hüttentechnische Vereinigung der Deutschen Glasindustrie e.V. (HVG – Research Association of the German Industry) in the scope of the “HyGlass” project.
As expected, the change of the fuel has an impact on the quality of the product (cf. also » Fig. 4), especially in the case of a very sensitive production process like glass manufacturing. The material analyses indicate that the hydrogen in the fuel often does not interact directly with the material being melted, that, however, there are indirect effects that can impact the product quality. For example, the higher water vapour content in the furnace atmosphere, which results from hydrogen combustion, leads to the removal of sulphur from the melt. This effect could be expected as it also occurs, for example, when the firing concept is changed from combustion with regeneratively preheated air to oxy-fuel combustion. Other effects are the possible reduction in the O2 content in the melt caused by the presence of CO and H2 in the furnace chamber. This can lead to discoloration of the glass [28].
Another observation in the tests was that with certain H2 admixture rates, more soot was formed, some of the soot then also being detected in the glass samples [29]. This is corroborated by the investigations of other researcher groups, who were also able to establish higher soot emissions in the combustion of natural gas-hydrogen blends [30], [31].
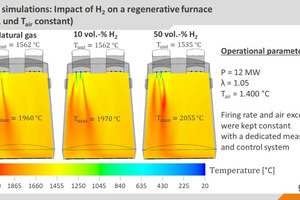
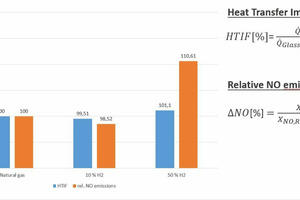
With the help of CFD simulations (CFD: computational fluid dynamics), the findings of semi-industrial investigations were extrapolated to real industrial plants. The simulations show that the effects of the fuel change on the plant operation are relatively low, if the changed fuel properties can be taken into consideration with an appropriate combustion control system, as can be seen in » Figs. 5 and 6, based on the example of a regenerative 12-MW glass melting tank. While higher local temperatures do result from hydrogen admixture (10 and 50 vol.-%) than in the reference case with natural gas, the impact on the heat fluxes into the glass bath or the temperature distributions tend to be minor.
This minimal change in the heat transfer into the glass bath (as an indicator of the energetic efficiency and product quality in the process) in comparison with the reference case with natural gas underscores the importance of suitable measurement and control systems to compensate for the impact of the fuel change. The change in the heat fluxes into the glass bath is defined here with the so-called Heat Transfer Impact Factor (HTIF), that is as the heat flux into the glass bath for a specific case, relative to the heat flux in the reference case, i.e. a HTID of 100 means that there is no change in the heat flux. However, a certain increase in the NOx concentrations can be expected due to the higher maximum temperatures in the system. It should be possible to manage these increases with modifications to the combustion processes.
Besides the technical aspects like combustion, glass quality and the formation of pollutant emissions, in the scope of the project, it was also investigated how far the supply of glass sites in North Rhine Westphalia can be realised based on locally installed electrolysers with wind and solar power. With help of a GIS analysis (GIS: Geographic Information System), glass sites in North Rhine Westphalia and their respective energy requirements were correlated with the sites and generation capacities of wind turbines and photovoltaic systems in a defined radius around the glass production sites. Based on the assumption that the wind turbines and PV systems within the radius produce power exclusively for the glassworks, which is converted into hydrogen with an electrolyser, it is possible to estimate how much electrolytically produced hydrogen can be potentially locally supplied for heating of the glass manufacturing sites. Here, typical operation runtimes and electrolyser efficiencies were assumed.
The GIS analysis shows that even under the these very optimistic assumptions, only for one in nine sites can complete local self-sufficiency be realised within a radius of 10 km, for a radius of 20 km, it is only three sites. This analysis underlines the importance of decarbonised energy supply infrastructures, especially for energy-intensive industries with continuous energy demand.
Ammonia as fuel
Besides hydrogen as a potential fuel for decarbonisation of industrial firing processes, ammonia (NH3) has also moved into the focus of interest in recent years. Ammonia is seen primarily as a hydrogen carrier, due to its higher volumetric calorific value and above all its high boiling point (-33 °C at atmospheric pressure), it brings considerable advantages with regard to intercontinental transport and the long-term storage of hydrogen. Studies by the International Energy Agency [32] predict that a considerable part of the globally traded hydrogen will probably be transported in the form of NH3 in the future, similar to LNG (Liquefied Natural Gas) today. Whereas a large part of this NH3 is split again in H2 and N2 on land in order to feed the hydrogen into the hydrogen grid, it is also possible to use ammonia directly as fuel. This has been discussed on the one hand for ship propulsion [33], [34], could, however, also be interesting for the supply of process heat, especially for sites that are not (yet) connected to a decarbonised energy supply infrastructure, but nevertheless want to decarbonise their processes.
Ammonia is not unproblematic as a fuel, key combustion-related challenges are the high necessary ignition energy, the narrow flammability range and the extremely low combustion velocities, which make it difficult to establish stable combustion. In addition, there is the danger of much higher nitrogene oxide emissions, as because of the nitrogen contained in the ammonia molecule, other NOx formation mechanisms, especially NOX formation based on the nitrogen bound in the fuel, become more relevant than in the combustion of natural gas or hydrogen. Up to now, there has been hardly any practical experience with the use of ammonia as fuel on an industrial scale, especially also in context of process heat supply. For that reason, in a series of projects (e.g. the recently launched project “NH3-Ziegel” [35]), GWI is currently analysing how using NH3 as a fuel can contribute to the decarbonisation of process heat in various industries. With the help of experimental investigations on laboratory and semi-industrial scale (up to 180 kW firing rate) and chemical kinetics simulations, it is considered how ammonia can be used sensibly and in an environmentally friendly way to provide process heat.
» Fig. 7 shows the initial findings of this work; on a non-premixed laboratory burner developed at GWI (firing rate 8 kW), the combustion of NH3/H2 blends is studied at various Swirl Numbers S [36]. As flame stabilisation is difficult in ammonia combustion due to the combustion properties of NH3, hydrogen is added to ammonia and a strong swirl is introduced into the air flow to assist flame stabilisation. The figure shows how both swirl and the H2 admixture impact the flame. In addition, it is clear that in the case of the pure H2 combustion, the flame is practically invisible, however, even just a small addition of NH3 leads to considerable changes in the radiation behaviour. This is caused by the NH2 radicals, which result from the oxidation of ammonia and give the flame its characteristic colour.
Summary
Because of the necessity of the decarbonisation of their processes, many industries, especially in the energy-intensive basic materials industries, face considerable challenges. A large part of the greenhouse gas emissions in these industries are produced by the combustion of fossil fuels for the supply of process heat. Besides the electrification of process heat (especially in the range of relatively low process temperatures), hydrogen from regenerative sources presents a promising option to supply process heat, especially high-temperature process heat, in a climate-friendly way.
As a fuel, hydrogen differs considerably from conventional fuels like, for example, natural gas. These special characteristics of hydrogen combustion must be taken into consideration on a fuel switch in thermal processing plants to minimise any negative effects, for instance in respect of product quality or pollutant emissions. Some of these effects have been presented with reference to examples from the glass industry.
Ammonia can also be an interesting option for the decarbonisation of industrial production processes, especially for sites that are not (yet) connected to a decarbonised energy infrastructure. Ammonia, however, comes with specific requirements with regard to stable and low-pollutant-emissions combustion. Corresponding research projects on the use of NH3 for process heating are currently underway, including at Gas- und Wärme-Institut Essen.