Raw material enthalpy of heavy clay raw materials (Part 1)
The raw material enthalpy dHR has a significant influence on the energetic balance of tunnel kilns in the heavy clay ceramics industry and can make up to 30 % of the total energy consumption of a kiln or cover up to 90 % of the total energy demand. Different methods are currently applied to determine the raw material enthalpy. For clay raw materials that do not contain any carbonates or organic components, the measured and calculated values agree comparatively well with deviations of maximum ±100 kJ/kg. If, however, the raw materials have a high carbonate and organics content, the results of the different methods of determination deviate considerably from each other. Up to now, it is not known which methods of determination actually provide practical values. This paper reports on comparative investigations on the raw material enthalpy in known raw materials. The different methods of determination (dynamic differential calorimetry, thermogravimetry, calorimetry, calculation based on the mineral content as well as on the content of water, carbonates and organic components) are presented and, based on the results, differences possible limitations to application and sources of error of the different methods are discussed.
1 Introduction
Energy savings and the associated cost reductions are a key issue in the clay brick and roofing tile industry. It is known that the enthalpy of the heavy clay raw materials to be fired has a significant influence on the energy consumption of a tunnel kiln, especially when the raw materials contain water, carbonates or organic components like wood, papermaking residues, bitumen or coal.
The following extreme examples show the maximum percentage that the heat-consuming (endothermal, positive value) and heat-releasing (exothermal, negative value) raw materials enthalpies can have in the energy consumption of conventional tunnel kilns for heavy clay ceramics (cf. [1–2]):
For backing bricks (BB), the raw material enthalpy has values between +600 kJ/kg (endothermal) and ‑1 000 kJ/kg (exothermal). For a specific energy consumption of 1 100 to 1 800 kJ/kg for BB, that corresponds to up to 90 % of fuel input of a tunnel kiln.
For facing bricks (FB) and clinker bricks, the raw material enthalpy has values between +600 kJ/kg (endothermal) and -300 kJ/kg (exothermal). For a specific energy consumption from 1 800 kJ/kg to 2 600 kJ/kg for FB and clinkers, this corresponds to up to around 30 % of fuel input of a tunnel kiln.
The heat-consuming and -releasing reactions of heavy clay raw materials are known to occur at different temperatures. Depending on the material composition, the heating rate, the brick format, the setting structure and accordingly the heat transfer mechanisms and the brick mass, these reactions can take place consecutively or they may overlap (cf. [3–5]). For this reason, the reactions take place during firing at different positions in the tunnel kiln. The kiln and its process control are adapted by plant engineering companies to the specific raw material to avoid reduced output or impaired quality. Besides the energy required for heating, the burner system must also put up the heat-consuming raw material enthalpy. Depending on the type and quantity of the organic components, the total released heat cannot, however, be utilized in the kiln. Depending on the reaction temperature of the organic components, the generated heat is partly released with the flue gases.
For the determination of the raw material enthalpy necessary for the kiln design, different methods continue to be applied. The experiences in recent decades in the Lingl laboratory have shown that especially with the presence of carbonates and organic components, the results of the different methods deviate from each other by one to two orders of magnitude (cf. »1 and »2) [6].
For the plant engineer, the significance of this issue lies in the energy consumption of tunnel kilns being increasingly linked to contractual penalties. In the past, this has even led to expert disputes between customers and plant engineering companies as a result of the application of different methods for determination and the consequently limited possibility for comparison of the measurement results. After all, for an additional energy consumption of, for example, +100 kJ/kg raw material enthalpy, a production rate of 200 000 t per year and a gas price of 27.35 € per MWh, the kiln operator must pay an additional energy cost of around 160 000 € per year (gas price from [7]).
For this reason, systematic laboratory analyses have been conducted with the hitherto-used determination methods on defined bodies and, with the calorimeter, another measurement method has been included in these analyses. Goal of the investigations was to compare the measurement results as well as to evaluate error and application limitations. The investigations were carried out as part of a master’s thesis in the “Ceramic Science and Engineerging” course of study at Koblenz University of Applied Sciences / University of Koblenz Landau (cf. [8]) and were made possible by Lingl Anlagenbau GmbH, for which the authors would like to express their special thanks at this point.
2 Fundamental concepts and methodology
During firing, the ceramic ware is heat-treated in accordance with a predefined firing curve. The thermal energy necessary for this is made up of two terms (Equation 1):
Heating of the ceramic ware to the appropriate temperature and
Enthalpy from the physical and chemical reactions in the material.
qG = cp * dT + dHR⇥(1)
qG The specific quantity of heat necessary for heat treatment of the ceramic ware in kJ/kg
cp Mean specific thermal capacity of the ceramic ware as a function of the temperature in kJ/kgK
dT Difference between the maximum firing temperature and entrance temperature of the ceramic ware fired in °C
dHR (Raw material) enthalpy of the physical and chemical reactions in the material in kJ/kg
During firing, heavy clay raw materials change their structure, forming amorphous and crystalline silicate phases. During this process, they release mainly the gases water vapour (H2O), carbon dioxide (CO2) and sulphur dioxide (SO2). The different reactions can consume or release heat (cf. »Table 1). With the addition of the temperature-dependent reaction heat of the different minerals, what is termed the raw material enthalpy results, which corresponds to the net calorific value Hu of fuels (cf. Section 2.3). The raw material enthalpy is relative to the specific dry mass of the material.
For heating and evaporation of the physically bound material moisture (adsorption moisture and water for shaping), energy is additionally necessary. This amounts at 25 °C to +2 442 kJ/kg water, i.e. +185 kJ/kg for heating from 25 °C to 100 °C and +2 257 kJ/kg for evaporation at 100 °C.
For determination of the raw material enthalpy of heavy clay raw materials, the following methods are currently applied:
Measurement:
With dynamic differential calorimetry -> institutes, university facilities and industrial laboratories
With a bomb calorimeter -> primarily institutes for fuels, including heavy clay raw materials
Calculation:
Based on the mineral content, determined by means of X-ray diffraction and simultaneous thermal analysis -> heavy clay institutes and plant engineering companies
From the mass loss of the thermogravimetry as well as from empirical values of differential thermal analysis or dynamic differential calorimetry -> institutes specializing in heavy clay raw materials
For all methods, the sample quantity should be as small as possible yet as large as necessary. On one hand, in thermal ana-lysis small sample quantities and typical heating rates lead to measurement results that can be evaluated. On the other hand, the sample quantity has to be large enough for the sample to be representative of the material even if contains coarse inclusions.
For a detailed description of the measurement methods, readers are referred to the relevant standards or literature. The following sections describe the essential aspects of the methods.
2.1 Differential Scanning Calorimetry DSC
In DSC (differential scanning calorimetry), the sample comminuted to a particle size smaller than 100 µm and a thermally inert material are each heated up in a crucible simultaneously in accordance with a defined temperature regime. The chemical and physical reactions taking place in the sample (cf. »Table 1) lead to heat being released or absorbed and therefore to a difference in temperature between the sample and the inert material. These temperature differences can be seen as a peak in the heat flow curve [9–11].
Calibration of the measurement device based on substances with a defined calorific value enables the calculation of the calorific values for the respective reactions from the peak areas of the heat flow curve. For area calculation, a baseline is defined. For chemical reactions and phase transformations in which the heat capacity of the sample changes, the baseline should be corrected with an inflection point [10]. In addition, the beginning and end of a reaction should be determined. To differentiate the reactions from each other, the TG curve measured at the same time and diverse software tools are used. Both require a lot of experience – both with the materials to be measured as well as with the evaluation software used.
The temperatures at which the reactions take place and the peak height are dependent on sample preparation (particle size, compaction in the crucible), the sample mass and the preheating rate. The thermal reactions of heavy clay raw materials are usually determined with a sample mass between 30 and 50 mg in platinum crucibles up to 1 050 °C. The preheating rates are 2 to 20 K/min. Lehnhäuser recommends a sample mass of 100 to 500 mg for heavy clay raw materials [11].
2.2 Thermogravimetry TG
In thermogravimetry, the sample is heated in a crucible in accordance with a predefined temperature regime. The sample mass or the mass change as a function of the temperature are determined [12].
»Table 1 shows the significant heat-consuming and -releasing reactions of heavy clay raw materials in the different temperature ranges. Because of the gases formed, a mass loss occurs. With the help of the differential TG curve (DTG), this can be demarcated more precisely and specified for the respective reaction. The determination of the individual mass losses requires the capture of a drive curve for the corresponding measurement conditions.
The right-hand column of the table contains the factors with which the raw material enthalpy can be calculated from the mass loss in the corresponding temperature range. These factors are empirical values from comparative measurements of the simultaneous thermal analysis of TG and DTA and DSC over the past decades [13].
2.3 Calorimeter, gross and net calorific value
The adiabatic bomb calorimeter was developed to determine the gross calorific value of fuels. It consists of an inner, water-filled calorimeter vessel with thermocouple and agitator as well as an outer, heated water jacket. The sample is weighed and placed into a crucible and this is suspended complete with ignition wire and combustion aids in the calorimeter vessel. After being closed, the bomb is filled with oxygen to a pressure of 30 bar and the remaining air is pressed out of the vessel. The ignition is effected from the outside with an ignition wire. The heat generated during combustion heats the water stirred by the agitator in the calorimeter vessel by a few kelvin. A thermocouple with high resolution measures this temperature change. With the simultaneous heating and cooling, the outer jacket undergoes corresponding temperature balancing so that no heat is released from the inside or absorbed from the outside (= adiabatic) [14].
The heat relative to the mass that the fuels release during complete combustion (exothermal) is termed the gross calorific value Ho. This is calculated according to [15] in the bomb calorimeter at constant volume from the measured temperature change dT (Equation 2).
Ho = (CK * dT – QZ) / mP⇥(2)
Ho Gross calorific value in J/g
CK Thermal capacity of the calorimeter system in J/K
dT Temperature increase of the calorimeter system in K
QZ Heat quantity of all combustion aids in J
mP Mass of the sample in g
The net calorific value Hu is calculated from the gross calorific value less the condensation heat of the water in the flue gas (cf. Equation 3, for heavy clay raw materials simplified with disregard of the volume work). This water can come from the available fuel moisture content and hydroxyl groups (OH groups) of water-containing minerals or be the combustion product of the hydrogen present in the organic components. The gross calorific value, that is the maximum utilizable heat of a fuel, is because of the addition of the condensation heat of the water always higher than the net calorific value (cf. values for wood and coal, Table 6).
Hu = Ho - [kH * H + 0.8 * (N + O) + kW * W] ⇥(3)
Hu net calorific value in J/g
kH specific heat of evaporation of the water formed from the hydrogen during combustion with +23.73 kJ per mass% hydrogen (25 °C)
kW specific heat of evaporation of water with +24.42 kJ per mass% water (25 °C)
H hydrogen content of the sample in mass%
N nitrogen content of the sample in mass%
O oxygen content of the sample in mass%
W water content in the sample in mass%
According to [15], the repeatability limit for individual measurements, one process operative and the same apparatus should be lower than 120 kJ/kg. Depending on the device, the detection limit lies between 200 kJ/kg and 300 kJ/kg dry substance (cf. [15]). The weight of the sample and combustion aids must be chosen such that the temperature increase in the calorimeter is about 0.7 to 1.3 times the temperature increase determined during determination of the thermal capacity of the calorimeter. As the temperature change of the water in the calorimeter vessel runs to around 3 K to 5 K as a result of combustion, the temperature is determined with an accuracy of at least 0.004 K [15, 17].
Compared with the gross calorific value of fuels, the raw material enthalpy of heavy clay raw materials is around 30 to 500 times lower. For this reason, for ignition and preheating to firing temperature, the low-calorific raw material sample is mixed with the measurement standard benzoic acid (gross calorific value
= -26 464 kJ/kg) in the mass ratio of 1.0 to 1.4 [18].
2.4 Mineral composition
The mineral composition of heavy clay raw materials can be determined with the help of X-ray diffractometry (XRD) and thermal analysis (DTA and DSC, TG as well as dilatometry). The quantitative determination of the clay mineral has been conducted in specialised laboratories for decades thanks to the development of highly sensitively instruments and the availability of appropriate calibration processes (External calibration method: calibration samples for individual clay minerals) and is described in [19–21]. To increase the accuracy of the XRD results, results of simultaneous thermal analysis (simultaneous determination of the DSC and TG curve) as well as dilatometry are used.
Organic components such as wood, coal or paper (papermaking residue) are determined separately in the form of organic carbon (Total Organic Carbon – TOC) or inorganic carbon (Total Inorganic Carbon – TIC) and as the total carbon (TC) during thermal treatment by means of infrared spectroscopy [22]. According to [23], from knowledge of the reaction heat of the individual raw material components and their percentages, the reaction heat per kg dry raw material results in accordance with Equation 4.
dHR, tro = ∑ri * dHi ⇥(4)
dHR, tro Raw material enthalpy, relative to the mass of the dry raw material in kJ/kg
ri Percentage of the raw material component i, dimensionless
dHi Reaction heat during the reaction of the raw material component i in kJ/kg dry
The raw material enthalpy of the firing goods is calculated with the help of the loss on ignition per kg fired according to Equation 5.
dHR, gebr = dHR, tro / (1 – GV)⇥(5)
dHR, gebr Raw material enthalpy, relative to the mass of the fired raw material in kJ/kg
GV Loss on ignition (as the sum of all volatile components, cf. »Table 1)
Besides thermal decomposition, new crystal phases can form. The reaction heat of the mineral phase formation is added to the resulting reaction heat (Equation 6).
qRE = dHR, gebr + ∑rj * dHj⇥(6)
qRE Resulting reaction heat, relative to the mass of the fired raw material in kJ/kg
rj Percentage of the newly formed mineral phase j in the fired material, dimensionless
dHj Reaction heat on formation of the mineral phase j in kJ/kg
Precondition for calculation of the raw material enthalpy from the mineral content is knowledge of the reaction heat of the individual raw material components. Hess’ theorem is used for calculation of the enthalpy changes on chemical reactions: “The enthalpy change dH of a total process is the sum of the enthalpy changes of the individual process steps.” The raw material enthalpy of a mineral can thus be calculated from the sum of the formation enthalpies of the final products less the sum of the formation enthalpies of the starting materials (Equation 7) [24].
dHi = ∑dH0B, E - ∑dH0B, A⇥ (7)
dHi Reaction heat on reaction of the raw material component i in kJ/kg dry
∑dH0B, E Sum of the standard formation enthalpies of the final products of a chemical reaction in kJ/mol
∑dH0B, A Sum of the standard formation enthalpies of the starting materials of a chemical reaction in kJ/mol
Precondition for the calculation is knowledge of the exact chemical formulae, the molar masses of all substances involved in the reaction and their standard enthalpies of formation dHB and the formulation of the chemical reaction equation. The chemical formulae, reaction equations and enthalpies of formation of non-clay minerals are largely known. In contrast, however, there is only a few and sometimes contradictory data on clay minerals as natural weathering products in literature. On the one hand, the availability of such data is made difficult because of the variability of the mineral composition. On the other hand, clay minerals seldom exhibit an ideal composition and are seldom present as single minerals.
»Table 2 shows the raw material enthalpies for selected minerals compiled from literature and calculated with Equation 7. With the values in the right-hand column, the raw material enthalpies from the XRD are calculated in this paper. They are defined on the basis of the works by [20, 23, 25–29].
The endothermal values specified for pure calcite and dolomite are too high for heavy clay raw materials, as in clays an additional exothermal formation of calcium silicates occurs above 850 °C. The new formations of the three main calcium silicates release heat as follows:
Wollastonite (CaO * SiO2) -715 to -760 kJ/kg ⇥[23, 28, 30]
Gehlenite (2 CaO * Al2O3 * SiO2) -381 to -500 kJ/kg ⇥[23, 28]
Anorthite (CaO * Al2O3 * 2 SiO2) -46 to -344 kJ/kg ⇥[28]
As experience with the firing of high-carbonate heavy clay raw materials shows, between 40 and 70 % amorphous phase and approx. 5 to 20 % calcium-aluminium silicates form, in this paper the values for pure calcite and dolomite are each corrected by -85 kJ/kg (exothermal). This value results from the authors’ own experience, based on an average of 11 % anorthite, 6 % gehlenite, 3 % wollastonite forming after firing. It should, however, be pointed out that silicate formation increases, the higher the firing temperature and the lower the cooling rate are and the finer the CaO present [23, 25, 30].
The various values for pyrite result from the different reaction products of pyrite decomposition to SO2 or SO3. The formation of SO2 or SO3 is dependent on pressure and temperature. The values for the clay minerals differ as they are based on different assumptions for the quantity of heat for dehydration [23, 27, 31]. In addition, which and how many new mineral phases are formed (exothermal reaction) and should therefore be taken into account in the calculations here as well depends on the firing regime.
Another simplified method for calculation of the raw material enthalpy uses only the three main heat-consuming and -releasing substances in the raw material: the chemically bound water (OH groups), the carbonates and the organic carbon (Equation 8).
dHR, tro = rW * dHR,W + rcarb * dHR,carb + rTOC * dHR,TOC⇥(8)
dHR, tro Raw material enthalpy, relative to the mass of the dry raw material in kJ/kg
rW, Carb, TOC Percentages of the raw material components in chemically bound water, carbonate and TOC, dimensionless
dHW, Carb, TOC Reaction heat on the reaction of the respective components in kJ/kg dry
With the help of TG, the water and carbonate contents can be determined. The organic (TOC) and inorganic carbon content (TIC of carbonates) are determined as described above. In »Table 2 the reaction heat used for calculation is specified for the water evaporation, carbonate deacidification and oxidation of the TOC.
3 Conducted investigations
3.1 Raw materials used
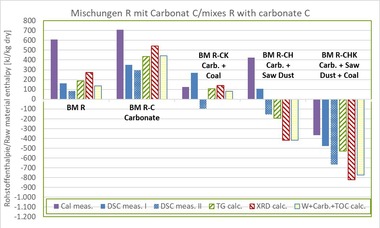
In the scope of this paper, the investigations were limited to a factory body for roofing tiles, BM R, without carbonates and organic components. Limestone flour Calcite C, wood sawdust H and coal K and their possible combinations, each with a field-relevant content, were used as additives. The composition of the mixes is shown in »Table 3.
The following properties of the raw materials were determined:
Process mix: Mineral content, grain size, loss on ignition, TOC, TIC and raw material enthalpy
Calcite: Mineral content, grain size, loss on ignition, and raw material enthalpy
Mixes: Grain size, loss on ignition, and raw material enthalpy
Wood and coal: Elemental analysis (chemical elements C, H, N, O and S), ash content, gross and net calorific value
3.2 Measurement methods applied
For production of the test specimens, the dry factory body was comminuted in a hammer mill to a particle size smaller than 2 mm and the coal screened to a particle size smaller than 1 mm. Only materials predried at 110 °C to a constant mass were used. Mixing was first performed in a dry process in a compulsory mixer and then with the necessary water required. The test specimens measuring W x H x L = 40 x 40 x 40 and 140 mm were produced in a de-aired laboratory extruder and then samples were taken to determine the mineral content, the grain size and the raw material enthalpy. The ceramics-related properties of the test specimens were also determined after drying and firing to 1 000 °C and combined DTA-gas expulsion measurements were performed, which, however, are not described in detail here.
The following measuring methods were applied:
Mineral content: quantitative X-ray analysis, clay minerals with triple powder analysis by means of XRD Stadi MP
Thermal analysis: STA 449 F3 Jupiter, Netzsch, sample mass 40 mg, heating rate 10 K/min
Particle size: Dry screening with mesh width 2 000 / 1 000 / 500 / 250 / 100 / 63 µm, < 32 µm with Sedigraph 5 120 II Micromeritics
Loss on ignition to 1 000 °C: gravimetric
Carbon content: Total carbon TC as well as inorganic carbon TIC in compliance with DIN ISO 10694, organic carbon
TOC = TC - TIC
Calorimeter: Gross calorific value after [14], [15] at 25 °C, adiabatic calorimeter C4000, IKA
Fuel analysis: Elemental analysis DIN 51724-3, DIN 51727, DIN 51732, DIN 51733, gross and net calorific value after [15] and -3 at 22 °C, adiabatic calorimeter C6000 with calorimeter bomb C6012, IKA, ash content DIN 51719, water content DIN 51718
3.3 Determination of the raw material enthalpy
The methods mentioned under Section 2 were applied for all mixes. Measurement of the DSC and TG curves as well as determination of the mineral content by means of XRD of the raw materials and mixes were performed at an institute specializing in clay minerals. The raw material enthalpies were calculated from the measured values of the TG and the mineral content and in a simplified process, as described in Sections 2.2 and 2.4.
Using a calorimeter, another institute specializing in heavy clay testing determined the gross calorific values of the raw material mixes. For this purpose, the raw material was dried at 55 °C to constant mass and the gross calorific value Ho,v determined as a the mean value from three individual measurements. The net calorific value Hu was calculated by the authors from the calorimeter gross calorific values and the contents of hydrogen H, nitrogen N and oxygen as well as the determined water contents from the TG (dehydration of the water from the clay minerals and hydroxides) in accordance with Equation 3. The gross and net calorific values of wood and coal were measured at an accredited institute specializing in fuels as described in Section 3.2.

![»1 Comparison of the raw material enthalpies dHR from thermal analysis measurements (DSC) with calculated values from the mineral content (XRD) and mass loss (TG), 33 data sets, TOC content ≤ 0.10 mass%, calcite ≤ 22 mass%, dolomite ≤ 28 mass% [6]](https://www.zi-online.info/imgs/1/6/8/5/9/1/1/tok_98cda42c6197f2804d7013535e29c989/w300_h200_x376_y226_FA_Lingl_Abb.1-9cb9fd4b357ed280.jpeg)
![»2 Comparison of raw material enthalpies dHR from thermal analysis measurements (DSC) with calculated values from the mineral content (XRD) and mass loss (TG), 128 datasets, TOC content ≤ 3.17 mass%, calcite ≤ 22 mass%, Dolomite ≤ 28 mass% [6]](https://www.zi-online.info/imgs/1/6/8/5/9/1/1/tok_4f8131c5a993aea922412e53ac7cd6eb/w300_h200_x376_y225_FA_Lingl_Abb.2-78c68c73b4dda952.jpeg)