Influence of mineral additives on the drying and sintering behaviour of brick bodies, Part 1
Drying is one of the most elementary processes in the manufacturing of ceramic products. With the removal of the water necessary for plastic shaping, the shaped product is transformed from a wet and plastic state into a dry state with a stable shape. As the evaporation of the water is an energy-consuming process, a reduction of the water needed for extrusion would therefore lead to an energy saving and, in addition, shorten the drying time.
1. Introduction
During shaping, the water serves as a sliding agent between the platelet-like clay minerals, rendering the clay plastic. Water is also responsible for the particles sticking to each other so the green product does not collapse as the content of mixing water decreases. Moreover, it fills the pores that form in the network of particles in the clay and makes up a considerable part of the total volume of the green product. With optimization of the particle size distribution (PSD) of a clay by means of precisely dosed mineral additives, these cavities can be minimized and the water content reduced.
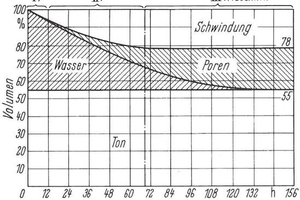
In »1, the drying curve is divided into three stages. In the first stage, the volume of the water removed corresponds exactly to the volume shrinkage of the green product, which is why no pores are formed in the green product. In the second stage, the volumetric shrinkage can no longer compensate for the quantity of water removed and the first pores are formed, the volume of which increases as shrinkage progresses. In the third stage, shrinkage has ended, but the green product still contains water, which during further drying is replaced by air, i.e. pores. Usually, in ceramics the first two stages are combined in the first drying stage, the end of which is marked by the end of shrinkage. The shrinkage is caused by capillary forces. As drying progresses from the outside inwards, a moisture gradient is formed in the green product and therefore also a shrinkage gradient, which leads to mechanical stresses in the green product. If these stresses exceed the strength of the green product, damage results in the form of deformation, providing plastic deformation is still possible, or in the form of drying cracks if such deformation is no longer possible. With a reduction of the water content or an increase in packing density, shrinkage is reduced, which lowers stresses in the green products during drying and thus mitigates the danger of damage.
The objective of this research project was the selective increase of the packing density of brick bodies by means of mineral additives so that on account of the decreased water requirement during extrusion, drying energy, drying time and drying shrinkage of green bodies are reduced. As mineral additives, by-products from the quarrying industry were used in order to minimize costs and utilize already available raw material potential.
2. Selection and characterization of the raw materials
For the research project, four mineral additives were selected which were sourced from quarries in Germany.
Additive 1: Weakly plastic crushed sand which can be classified as being part of the Rotliegend
Additive 2: Mica-bearing feldspar sand recovered during granite processing
Additive 3: Weakly plastic rock flour formed during andesite processing
Additive 4: Weakly plastic rock flour with a high content of phyllosilicates, produced during the processing of diabase
Additives 2 to 4 are filter dusts collected in the dedusting system during crushing of extracted rock. These have the advantage the quarrying companies themselves have no use for them so they are commensurately cheap to buy. One disadvantage is their PSD. As the additives are all produced during dedusting, they have a very similar PSD, which also differs very little from that of clays, which is why an increase in packing density can hardly be achieved with their addition. For this reason, as the fourth additive, a crushed sand with a very narrow PSD and a comparatively large mean particle size was chosen (Additive 1). This can be used to effectively influence the PSD. As brick bodies, widely differing product groups were selected, including two backing brick bodies (HLZ 1 and 2), two roofing tile bodies (DZ 1 and 2), a clay paver body (PK) and a facing brick body (VMZ).
The filter dusts differ widely depending on the original rock and also exhibit a clearly different mineralogical and chemical composition from that of the clays (see »Table 1). Of all the additives, Additive 4 contains the most phyllosilicates, which consist of almost exclusively of clay minerals (smectite and chlorite). Additive 3 also contains relatively quantities of smectite and chlorite, whereas the other two additives contain predominantly mica as a phyllosilicate. The different clay mineral contents are already a first indication of possible differences with regard to the sintering activity of the additives. Additives with high clay mineral content will demonstrate reactions already at relatively low temperatures compared with additives that contain more tetrasilicates (quartz, feldspar). Quartz is present in the additives in sometimes considerable percentages. Besides these, various feldspars are present in widely varying quantities. Further in Additive 4 the inosilicates augite, amphibole and prehnite, which are unusual compared with clays, can be detected. Augite is also present in Additive 3. In three of the four additives, there are also carbonates in the form of calcite and dolomite. Further, in Additives 2, 3 and 4, iron minerals such as haematite, magnetite and goethite are detected. In addition, these additives contain a small quantity of the calcium phosphate mineral apatite. Traces of pyrite are present in two additives. In addition, small quantities of organics are detectable in all additives.
The chemical composition of the additives reflects the mineral phases they contain (see »Table 2). On account of its high content of quartz, Additive 1 has a very high content of SiO2. The Al2O3 content is low as the additive contains few phyllosilicates and hardly any feldspar. Additive 4 contains the least SiO2 as only a very small quantity of quartz is present. The feldspar content is low compared with Additives 2 and 3, which is why the Al2O3 is lower, despite the higher content of phyllosilicates, than in these two. Fe2O3 comes primarily from the iron compounds haematite, magnetite and goethite; moreover, especially in Additive 4, from the chlorite and inosilicates. Calcium is contained in the carbonates, the anorthite-containing feldspars such as plagioclase or labradorite and the inosilicates. Magnesium can be found mainly in chlorite, augite and amphibole.
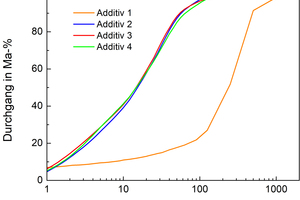
In the PSD, the different “production process” of the additives can be clearly identified. Additives 2 to 4 are filter dusts, which are produced in the dedusting systems of the quarries. As a result, they have a quasi-identical and tendentially wide PSD (see »2). Additive 1 is a crushed sand with a much coarser and narrower PSD.
As the analysis results in »Table 1 show, the phyllosilicate content of the clays vary between 42 and 65 %. In all clays, mica and its weathered product illite can be detected in various quantities, although mica is not a clay mineral and, in contrast to illite, this does not contribute to the plasticity of the clay. Illite-smectite alternating strata, smectite, kaolinite and chlorite are present as other clay minerals. Especially the clay VMZ, but also the two roofing tile clays have a high content of kaolinite. Among the tectosilicates, as usual, quartz is the determining component. In addition, diverse feldspars are present. Carbonates can only be detected in the backing brick clays, clay HLZ 2 having a somewhat higher carbonate content, which consists of 73 % calcite and 27 % dolomite. HLZ 1 exhibits almost the same percentages of calcite and dolomite. Apart from clay VMZ, all clays contain a certain quantity of iron-bearing minerals (goethite, haematite). The clay VMZ, on the other hand, is free of iron and is the only one to contain a small quantity of anatase (TiO2).
The results of chemical analysis correspond to the mineralogy of the clays (see »Table 2). The content of Al2O3 correlates with the quantities of phyllosilicates and feldspars contained in the clay as only these contain Al2O3. Accordingly, clay PK has the highest Al2O3 content. Although the clays VMZ, DZ 1, DZ 2 and HLZ 1 have similar contents of phyllosilicates and feldspars, they differ widely in Al2O3 content. This is caused by the different content of kaolinite, which is a particularly Al2O3-rich clay mineral. The SiO2 content in the clay is determined mainly by the quartz content besides the quantity of phyllosilicates and feldspars. Accordingly, there is a correlation here. The main content of the Fe2O3 is in the iron-bearing minerals haematite and goethite. In addition there are iron atoms in the lattice of diverse phyllosilicates. Clay VMZ contains only a small content of Fe2O3, as the clay is obviously a kaolin. Large quantities of CaO and MgO are found only in the backing brick clays and are caused by the calcium and magnesium carbonates contained in them. The origin of the K2O lies primarily in the micas and the orthoclase. Na2O is only contained in very small quantities and comes mainly from the feldspars and to a small extent from the phyllosilicates.
The PSD of the clays is shown in »3. The curves show a curve typical for clays, that is an especially wide distribution without pronounced peaks. »Table 3 shows the mean particle diameter D50 and the clay mineral fraction of the respective clays. Mean particle diameter means that 50 % of the particles are smaller than this value. The particle sizes < 2 µm are described as the clay mineral fraction. This range is largest in the VMZ clay as it contains large quantities of illite and kaolinite. For this reason, this clay also shows the smallest D50. The roofing tile clays have similar mineralogical compositions, however, the DZ 1 clay has a lower D50. This indicates that the tectosilicates are present in a finer form here than in clay DZ 2. For the vertically perforated brick clays, an interpretation is difficult as in contrast to the other clays, they contain considerable quantities of carbonates, which can also be concentrated in the clay mineral fraction. Things are different in clay PK. Although this has the highest percentage of phyllosilicates, it shows the lowest content of clay minerals. This can probably be attributed to the fact that the dioctahedral micas are in fact micas (and not illites), which in contrast to the illites are not counted as clay minerals. In addition, this clay is a shale, which has forfeited part of plasticity as a result of consolidation.
3. Modification of the clay bodies
The additives are to be combined with the clays such that the green products are extruded with high packing density and correspondingly low water content, which, however, after firing exhibits the same strength as the original brick bodies. That means on the one hand that the content of additives should be as high as possible to increase the packing density, but on the other hand the clay content is reduced as a result, and possibly the sintering activity, which depends on the mineralogical composition of the additive and the firing temperature of the bricks. As a compromise, the content of the additives was limited to 30 mass%.
To increase the packing density, various mixes of clays and additives were prepared, the optimization of which is based on the Dinger-Funk equation:
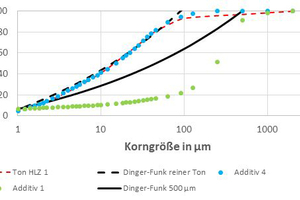
P(D) represents the percentage of the particles that are smaller than the diameter D. Dmin and Dmax stand for the minimum and maximum particle diameter. n is a fit parameter, which influences the gradient of the curve. Dinger and Funk were able to prove that a maximum packing density can be achieved with only n ≤ 0.37 (Funk und Dinger 1994). In this research project, for optimization of the packing density, calculations were performed with a fit parameter n = 0.2, as the best results were achieved with this value in AiF project 17570 N. Dmin and Dmax were defined based on the PSD of the raw materials, which was determined by means of laser granulometry for the size range < 125 µm and wet screening analysis for the range > 125 µm. Dmin was defined such that the point of intersection of the calculated Dinger-Funk curve with the y-axis lay in the range of the additives and the clay. For the maximum particle size, the particle diameter of the coarse grain that is still present in relatively large quantities in the mix was used. »4 describes this procedure based on clay HLZ 1. The dashed Dinger-Funk curve was calculated with a Dmax of 90 µm, as above this particle size, the PSD of the clay levels out considerably. Expressed in other words, only less than 8 mass% are coarser than 90 µm, but are distributed over a particle size spectrum from 90 to 2 000 µm and therefore have hardly any influence on the packing density. For this reason, a calculation with values > 90 µm makes little sense. The figure also demonstrates the fundamental problem in the optimization of a clay, as it becomes clear that the clay shown already agrees very well with the Dinger-Funk curve and therefore leaves hardly any potential for improvement. Moreover, the PSD of Additive 4 corresponds fairly precisely to that of the clay, which is why the PSD of the clay can hardly be changed with this additive. As already mentioned, with Additive 1, a crushed sand with much coarser PSD was selected for this reason. The use of this crushed sand leads to the Dinger-Funk curve shifting to the right as Dmax increases to 500 µm. With the coarser particles, the packing density can now be increased compared to the pure clay as a large sphere always takes up a smaller space than several small spheres with the same total volume.
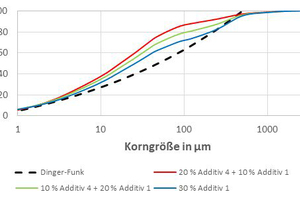
The three other additives, however, should not be discarded. For this reason, the PSD of various mixes of the filter dust and crushed sand was calculated (see »5). The higher the content of Additive 1, the better the PSD agrees with the Dinger-Funk curve. However, this additive is, compared with the other additives, a coarse crushed sand with a high content of quartz, i.e. the sintering activity is probably weaker. The decision was therefore taken to add a total of 30 mass% mineral additive, consisting of 20 mass% crushed sand (Additive 1) and 10 mass% of the respective filter dust (Additive 2 to 4).
In »Table 4, the brick bodies produced from these with the abbreviations used in this project are listed. For the Body PK, the composition was varied and 30 mass% of Additive 1 to 4, respectively, added to the clay. In this way, the different effects of the fine filter dust and the coarse crushed sand on the packing density and the physical properties in a stronger form were to be analysed.
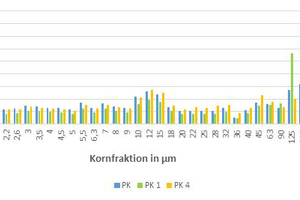
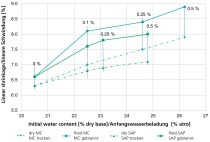
For each mix, the PSD was calculated based on the individual raw materials and this subsequently divided into particle sizes, which served as the input parameters for a program to calculate the packing density. »6 contains the particle size fractions of the basic body PK and the modified brick bodies as a bar chart. It can be seen clearly that with the crushed sand (PK 1), the coarse fraction has been strongly enriched, whereas the filter dust even reduces the percentage of coarse fraction and instead increases the percentage of the mean particle fractions (PK 4). In »Table 4, the calculated packing densities for the brick bodies are listed. As expected, already the pure clays or brick bodies achieve very high packing densities on account of their wide PSD. As a result of the mineral additives, the packing density is nevertheless increased by 2 to 3 %. One exception here is again Body PK. With the 30-% addition of the crushed sand, the packing density is increased by 3.5 %, whereas the addition of 30 % filter dust does not lead to any change in the packing density. The clearly indicates again that an increase in the packing density is only possible when the D50 of clay and additive differ clearly.
4. Characterization of the wet brick bodies
The bodies were all extruded with a Pfefferkorn deformation of 25, apart from Body HLZ 1 12, which was unintentionally set to a value of 24. As can be seen in »Table 5, the moisture content for pressing the modified brick bodies (except Body HLZ 1 12) is lower than that of the basic body. This is probably caused by the higher packing density. It is, however, generally known that with the addition of an opening or grogging agent, the water required for extrusion decreases. This is attributed to the property of clay minerals to adhesively bind large quantities of water on account of their large specific surface area and high surface charge. A decrease in the clay mineral content consequently effects a lower moisture content needed for extrusion.
The modified bodies tend to exhibit a higher pressure head pressure than the basic bodies, which can be probably attributed to the lower moisture content or the coarse crushed sand. Possibly, as a result of this, the sliding capacity of the clay mineral particles is impeded. An exception is Body DZ 2. Here, the pressure head pressure is reduced compared to that for the basic body despite the somewhat lower water content. The tensile strength of the moist brick bodies tends to be higher than that of the basic body and correlates very well with the pressure head pressure. This result fits with the assumption that the sliding capacity of the clay mineral particles is reduced with the addition of the additives as the tensile failure ultimately is down to the plastic flow of the body, i.e. in this case, there is no brittle fracture as in the case of dried or fired specimens, but when the yield point is exceeded, the body starts to deform plastically and ultimately tears.
»Table 6 lists the crucial parameters for water transport during drying, i.e. moisture conductivity and water vapour diffusion factor. The moisture conductivity tends to be increased by the additives, however, sometimes even a lower moisture conductivity than that of the basic body was measured, which may be down to the scatter of the measurement method. Moisture conductivity and water vapour diffusion resistance factor do not correlate with the mean pore diameter or the calculated packing density, which is why it can be assumed that the latter is only of minor significance for the drying velocity. However, it is noticeable that moisture conductivity and water vapour diffusion resistance correlate with each other positively, i.e. basic bodies with a high moisture conductivity have a relatively high water vapour diffusion resistance factor. This may be attributed to the mineralogy of the clays, which contain different percentages of swelling and non-swelling clay minerals. (Kohno 2020) was able to prove that a negative linear relationship exists between the swelling pressure (pressure generated when clays absorb water) and the moisture conductivity of the clay minerals, i.e. swelling clay minerals effect poorer moisture conductivity. This is caused by the volume increase of the clay minerals during water absorption, which leads to closing of the pore channels. Especially smectites, which consist mainly of montmorillonite, have a negative effect here. While apart from VMZ body, all clays have smectite or illite-smectite alternating strata (the percentage of smectite in the alternating strata being unknown), but if only the pure smectite content is taken into account, the smectite-bearing basic bodies HLZ 1 and 2 and DZ 2 have lower moisture conductivities and water vapour diffusion resistance factors than the three other smectite-free bodies. Although the mineral additives sometime contain considerable quantities of smectite, no systematic influence on the moisture conductivity can be identified.
On account of its swelling capacity, smectite also has a very large specific surface area, as a result of which it can bind a lot of water vapour (see »Table 7). This is possibly the reason for the lower water vapour diffusion resistance of the smectite-bearing clays. The large surface area of the smectite in combination with its very high affinity for water molecules ensures that the clay soaks up water molecules like a sponge. A concentration gradient within the clay, as produced during the attempt to measure of the water vapour permeability, is there quickly balanced out as the water molecules are quickly transported from the wet surface to the dry surface. For this reason, determination of the water vapour diffusion resistance factor is possibly also suitable for characterizing the drying sensitivity of brick bodies. Besides the mineralogy, other parameters have an influence on the drying sensitivity like, for example, the PSD, the drying shrinkage or the tensile strength of the wet and dry clay. (Part 2 of this article will follow in ZI 2/2022.)