Raw material enthalpy of heavy clay raw materials (Part 2)
The raw material enthalpy dHR has a significant influence on the energetic balance of tunnel kilns in the heavy clay ceramics industry and can make up to 30 % of the total energy consumption of a kiln or cover up to 90 % of the total energy demand. Different methods are currently applied to determine the raw material enthalpy. For clay raw materials that do not contain any carbonates or organic components, the measured and calculated values agree comparatively well with deviations of maximum ±100 kJ/kg. If, however, the raw materials have a high carbonate and organics content, the results of the different methods of determination deviate considerably from each other. Up to now, it is not known which methods of determination actually provide practical values. This paper reports on comparative investigations on the raw material enthalpy in known raw materials. The different methods of determination (dynamic differential calorimetry, thermogravimetry, calorimetry, calculation based on the mineral content as well as on the content of water, carbonates and organic components) are presented and, based on the results, differences possible limitations to application and sources of error of the different methods are discussed. Read part 2 here (part 1 in ZI 4/2021).
4 Results and discussion
4.1 Properties of the raw materials
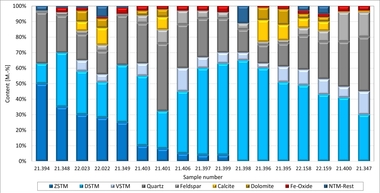
The properties of the raw materials used are shown in »Tables 4, 5 and 6.
4.2. Determined raw materials enthalpies
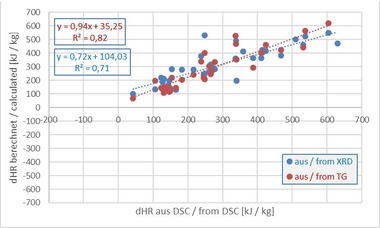
The measured and calculated raw material enthalpies of the mixes analysed are shown in Table 7. To show the wide scattering of the individual values, the mean value and the standard deviation of the results for all five methods were calculated.
The results show that the raw material enthalpies determined in the calorimeter, with the exception of the coal-containing mixes BM R-K and BM R-CK, are considerably higher than the enthalpies determined with other methods (consideration of the values here with the respective sign, cf. »3 and »4). For BM R-K mix, the value determined in the calorimeter is much lower than the other measured values. In contrast, the calorimeter value for the BM R-CK mix is in the same order of magnitude as the other values. For this reason, in the following, the disproportionate values for each measurement series are considered separately.
»Table 7 as well as »3 and »4 contain the results of the same DSC measurement, which, however, was evaluated by different processors. Processor I had no knowledge of the mix composition. Processor II, on the other hand, knew the components and their percentages in the mixes. As in test laboratory practice, the composition of the raw materials is not usually known, in the following, only the results from Processor I are considered.
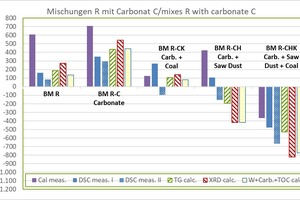
»3 shows the determined raw material enthalpies of the carbonate-containing mixes compared with the BM R starting mix. The abbreviations used correspond to »Table 7.
Results for BM R:
the dHR from the KAL amounts to +610 kJ/kg and is comparatively high
the dHR without KAL lies between +136 and +271 kJ/kg (mean value +189 ±51 kJ/kg), the lowest value being from WCC and the highest value from XRD
Results for BM R-C:
the dHR from the KAL amounts to +708 kJ/kg and is comparatively high
the dHR without KAL lies between +351 and +543 kJ/kg (mean value +443 ±68 kJ/kg), the lowest value being from DSC and the highest value from XRD
Results for BM R-CK:
the dHR from the DSC amounts to +268 kJ/kg and is comparatively high
die dHR without DSC lies between +81 and +139 kJ/kg (mean value +112 ±22 kJ/kg), the lowest value being from WCC and the highest value from XRD
Results for BM R-CH:
the dHR of DSC and KAL is positive (endothermal) and comparatively high,
the dHR of DSC amounts to +107 kJ/kg and the dHR from the KAL amounts to +424 kJ/kg
the dHR without DSC and KAL is negative (exothermal) and lies between -420 and -193 (mean value -344 ±107 kJ/kg), the lowest value being from WCC and the highest value from TG
Results for BM R-CHK:
all values are negative (exothermal) and are comparatively widely scattered
the dHR lies between -823 and -369 kJ/kg (mean value -597 ±178 kJ/kg),
lowest value from XRD and highest value with KAL
the dHR from the KAL amounts to -369 kJ/kg and is still comparatively high
the dHR without KAL lies between -823 and -477 kJ/kg (mean value -651 ±150 kJ/kg)
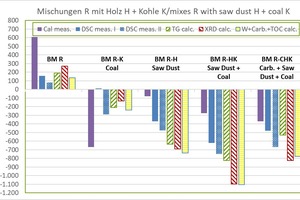
»4 shows the determined raw material enthalpies of the organics-containing mixes compared with the BM R starting mix.
Results for BM R-K:
the dHR from the KAL amounts to -669 kJ/kg and is comparatively low
the dHR without KAL lies between -240 and +12 kJ/kg (mean value -143 ±97 kJ/kg), the lowest value being from WCC and the highest value from DSC
Results for BM R-H:
the dHR from the KAL amounts to -78 kJ/kg and is comparatively high
the dHR without KAL lies between -735 and -369 kJ/kg (mean value -607 ±142 kJ/kg), the lowest value being from WCC and the highest value from DSC
Results for BM R-HK:
the dHR from the KAL amounts to -276 kJ/kg and is comparatively high
the dHR without KAL lies between -1103 and -621 kJ/kg (mean value -911 ± 201 kJ/kg), the lowest value being from WCC and the highest value from DSC
Results for BM R-CHK:
all values are negative (exothermal) and are scattered comparatively widely
the dHR lies between -823 and -369 kJ/kg (mean value -597 ±178 kJ/kg),
the lowest value being from XRD and the highest value with
KAL
the dHR from the KAL amounts to -369 kJ/kg and is still comparatively high
the dHR without KAL lies between -823 and -477 kJ/kg (mean value -651 ± 150 kJ/kg)
4.3 Discussion
The DSC evaluated by Processor I returns raw material enthalpies that with the exception of BM R, the pure roofing tile mix, differ sometimes considerably from the results of the calculation methods and from the calorimeter. For the mixes containing organics, the enthalpies from DSC I are much higher than the calculated values. With the exception of the coal-containing mixes BM R-K and BM R-CK, the results are, however, lower than the calorimeter values. The raw material enthalpies of the DSC evaluated by Processor II are all lower than for Processor I. For the mixes – with the exception of the coal-containing mix BM R-CK, the enthalpies from the DSC II are similar to the calculated values. All results are lower than the calorimeter values. The results show that the raw material enthalpies determined in the calorimeter are, with the exception of the two coal mixes BM R-K and BM R-CK, much higher than for the other methods. The three calculation methods return raw material enthalpies with approximately the same order of magnitude. This is not surprising as these three methods are based on the same theoretical assumptions with regard to dewatering, decarbonatization and oxidation. With the simplified WCC calculation, mostly lower enthalpies were determined that with the calculation from XRD or TG. As the results shown sometimes differ considerably, especially for the combination of carbonates and organics, and the question remains with regard to which method ultimately returns a “correct” result, possible sources of error are discussed in the following.
4.3.1 Sampling, TOC determination
For the results comparison of the measurement and calculation methods applied, representative sampling is of crucial importance. Heavy clay raw materials are intrinsically inhomogeneous. Already in the clay pit or during sampling there can be wide differences in the composition of the raw material, and individual particles like, for example, lumps of limestone, fibrous organic components or clay[A1] cavities are not representative of the raw material overall. It could be shown that especially small changes in the TOC or carbonate content have a disproportionate effect on the overall raw material enthalpy. For the tests, homogenized sample material was used, as described in Section 3.2.
4.3.2 Thermal analysis DSC, TG
Temperature-resolved thermal analysis is the only one of the methods presented here to make the reactions occurring during preheating visible. Determination of the raw material enthalpy with the help of DSC and TG is dependent on the following factors, which have a significant influence on the result:
The sample quantity used is small and therefore not very representative for heavy clay raw materials, so the requirements for sampling are high.
Depending on the analyser, the sample preparation, the sample quantity and the particle size as well as the preheating rate, reactions during thermal analysis take place at different temperatures than in the real firing process. For instance, reactions that take place consecutively during thermal analysis may overlap in the real firing process and impede each other, for example degassing and oxidation. It is not known how far this affects the resulting raw material enthalpy.
The measurements are conducted mostly up to 1050 °C. Higher temperatures and the formation of new minerals or melt phases are not taken into consideration. Depending on the firing regime, new mineral phases and melts can form, which additionally release or consume heat, These phenomena are also dependent on sample preparation, sample quantity and particle size as well as the heating rate.
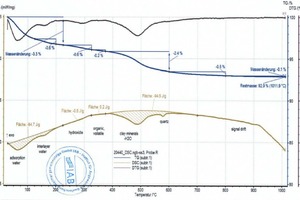
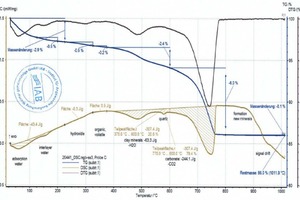
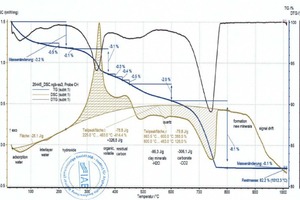
During heating, the key thermophysical properties of the sample (mass, thermal capacity, thermal conductivity, porosity and thus the gas permeability) change. As a result the base line is shifted. This shift is stronger, the greater the reactions in the material are. This means that the base line for pure clays deviates from that of a clay with carbonates or organics and therefore must be set more or less subjectively. This is shown by the two diagrams (»5) for the BM R and BM R-C mixes (bold beige curves and peak areas). The two mixes differ only by the percentages of the BM R mix and carbonate (cf. »Table 3). The base lines, each seen as thin, beige lines above the cross-hatched areas, are almost identical up to 600 °C. Above 600 °C, in the BM R-C mix, the carbonates are neutralized and the base line differs considerably from the BM R mix.
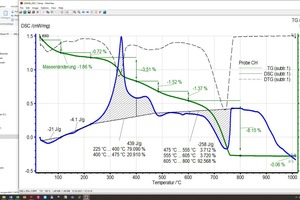
The raw material enthalpy is calculated from the peak areas of the individual reactions. For the definition of these areas, the processor defines the temperature ranges in which the individual reactions start and end. The TG curve and software tools facilitate demarcation of these areas considerably. However, setting of the base line, the curve selection for the area determination and the determination of the beginning and end of a reaction are always dependent on the processor’s level of knowledge and their knowledge of the material composition, especially when overlapping reactions occur, as in the case of organic burnout or the decomposition of carbonate and clay mineral. This is shown by the diagrams (»6) for the BM R-CH mix after evaluation by the different processors, In this example, the raw material enthalpies differ by 263 kJ/kg.
If several overlapping exo- and endothermal reactions occur, the summary raw material enthalpy depends on whether the baseline in the diagram is set further up (increase of exothermal and decrease of the endothermal percentage) or further down (increase of the endothermal and decrease of the exothermal percentage). The evaluation of the reactions – individual or summary evaluation – influences the summary raw material enthalpies. Even with precise knowledge of the material composition, no 100-% reliable evaluation is possible and the result should only be regarded as an order of magnitude. One remedy here can be a complex calibration of the DSC with the same material prefired in different temperature steps (e.g. in 100-°C steps).
4.3.3 Calorimeter
Determination of the raw material enthalpy with the help of a calorimeter is dependent on the following factors, which can significantly influence the result:
The calorimeter returns a summary measured value that describes the energetic change of the material from the starting to the final state after complete combustion of the added aids and the organics in the material. Individual reactions as in the thermal analysis are not detected. With the deduction of the gross calorific value of the aids used and with consideration of the thermal capacity of the calorimeter device, the gross calorific value of the material is calculated. As a consequence of the complete combustion of the benzoic acid and the combustion aids[A1], the ceramic sample material can melt (melting enthalpy). In addition, at elevated temperatures and high pressure, the formation of new minerals is possible (mineral formation enthalpy). These enthalpies are besides the values listed in »Table 1 also contained in the measured value and not known. According to [32], on account of the much larger sintering and vitrification reactions compared to the normal firing process, the determined gross calorific value is 21 to 42 kJ/kg too low.
The determined gross calorific value is converted into the net calorific vale. The necessary contents of the chemical elements H, C, O, N, S present were not measured in this work, but only calculated from the wood and coal components. The associated error in the conversion to the net calorific value is, however, on account of the low quantities of H, C, O, N and S in the BM R pure factory mix, estimated to be low.
In the literature, gross calorific values Ho from the calorimeter have often been equated with raw material enthalpies (calculated or measured by means of DSC) or the net calorific values. This leads to misassumptions for the firing process as here only the net calorific value can be used or the corresponding heat must be supplied. For the mixes tested, the difference between Hu and Ho is 142 to 217 kJ/kg. For wood and coal without clay, the different already amounts to 1110 to 1370 kJ/kg.
The calorimeter was developed to determine the gross calorific value of fuels. This value is an absolute value one to two orders of magnitude higher than the raw material enthalpy of heavy clay raw materials. To detect the low enthalpies of heavy clay raw materials in the calorimeter, benzoic acid is added to the material sample. If the quantities of material sample and benzoic acid in addition to the combustion aids and the associated enthalpies are put into relation with each other, it is observed that a material sample with for example -200 kJ/kg has a percentage of 0.7 % of the total enthalpy determined in the calorimeter, which leads to a temperature increase of 0.019 K. In contrast, the percentages of the combustion aids in the total enthalpy amount to 66.6 % for the benzoic acid, 26.5 % for the sachet and 6.1 % for the ignition wire. It appears questionable whether the measurement system can correctly capture these low values (cf. reproducibility scatter and detection limit).
Measurement in the calorimeter is conducted in pure oxygen atmosphere at a pressure of 30 bar and therefore does not correspond to real conditions in a tunnel kiln. Here a slight negative pressure of a few millibars prevails and the oxygen content is lower than 21 %. With the high pressure in the calorimeter, the reactions can also take place at other temperatures or the quantities of the reaction products can change.
4.3.4 Calculation from XRD, TG, WCC
The calculation of the raw material enthalpy from the mineral content comes with immanent potential errors so that the determined values can only be understood as an order of magnitude. The following sources of error deserve explicit mention [21, 23]:
In the qualitative and quantitative determination of the clay minerals by means of XRD, sufficient experience as well as standardized comparative samples of the pure minerals are necessary. For this reason, the results for the mineral content can differ enormously from one laboratory to another and lead to different results for the raw material enthalpy.
Uncertainties in the mineralogical analysis of the starting raw materials, variations in the body composition, sources of error in the analysis instruments and imponderabilities in the evaluation methods cannot be precluded.
Despite belonging to the same class, mineral phases, especially the clay minerals, can differ in their chemical composition, layer charges in the degree of disorder and, on account of lattice deformations, in their reaction heat from the values for dHi specified in the literature.
The influence of temperature, time and grain structure as well as the different reaction processes and possible differences in the heat tone in hydrothermal, reducing and oxidizing conditions are not known or only incompletely known. The influence of the atmosphere is not taken into consideration.
Interactions of the minerals and overlapping of reactions cannot be incorporated on account of the multicomponent system in heavy clay raw materials.
Sintering reactions and the formation of new minerals have so far been neglected in heavy clay ceramics on account of equilibria not being established in a short firing time, high mass throughputs or low firing temperatures well below the sintering point. They should, however, be taken into consideration in the case of long firing times, at firing temperatures > 1000 °C as well as low cooling rates.
The values used for dHi in the calculations are taken from the literature. Determination methods and conditions are not known and a reproducibility for the conditions of heavy clay firing has not been proven.
The empirical values for calculation of the raw material enthalpy from the temperature-resolved mass losses (TG curve) are based on measured values from the thermal analysis. These measurement results can come with errors on account of the subjective demarcation of the reactions (see DSC error analysis).
5 Conclusion and outlook
The methods currently applied to determine the raw material enthalpy dHR of raw materials used in the heavy clay industry are described in depth. Taking the example of a roofing tile body to which lime flour, sawdust and coal had been added, the methods were applied and the results compared.
It was shown that
Every method exhibits fundamental sources of error, which, depending on the method, differ widely from each other.
It could not be established which of the methods applied returns the “correct” results for the firing process in the tunnel kiln.
Comparability of the results of different methods is therefore only possible to a limited extent because of these reasons.
The simplified calculation from the contents of water of crystallization, carbonates and TOC as well as the calculation from the temperature-resolved mass losses of the TG for the materials investigated returns similar values to those of the more complex calculation based on the individual minerals.
The biggest differences lie between the calorimeter values and the values determined with other methods.
The values from the calorimeter for the mixes with wood and coal do not add up as would have been expected and as established with the other methods.
The difference between Ho and Hu for the investigated mixes in the calorimeter is comparatively large. The measurement result (gross calorific value Ho) must be converted to the net calorific value Hu. For this, the contents of hydrogen, nitrogen and oxygen must be available, which, in the authors’ experience, are not generally determined.
The experience so far confirms that, if coal, wood and carbonates are presented, the calculated and measured raw material enthalpies deviate further from each other than for low-carbonate and low-organics raw materials.
Even low quantities of organic carbon, which are typically added to clay backing bricks or blocks (HMz) for the purpose of porosification and the reduction of energy consumption and to facing bricks (VMz) to obtain certain colour shades, have a much higher influence on the results of the different methods than carbonate neutralization and the clay mineral reactions.
In the evaluation of DSC measurement, knowledge of the material composition can return different results than if no knowledge of the material composition is available
The investigations were, apart from the measurement in the calorimeter, individual measurements. This is standard practice despite wide variations in the composition of heavy clay ceramic raw materials. Recommended for further work is the verification of the established results and their underpinning with tests on other raw materials. The results therefore leave plenty of scope for future work. The question of which method returns correct values with which raw materials therefore requires further systematic investigation.
Ton-Keramik-Umwelt TKU (1990–2011) sowie Institut für Angewandte Bauforschung Weimar IAB seit 2011
Teil 3: Verfahren mit adiabatischem Mantel. Beuth Verlag Berlin 2005
Teil 1: Allgemeine Angaben, Grundgeräte, Grundverfahren. Beuth Verlag Berlin 2000
ZI-Jahrbuch (1993), S. 70–111.